The position of an element in. There are different types of hybridization-based on the mixing of the orbitals.
Electron Configuration Detailed Explanation Filling Of Orbital Representation Of Electronic Configuration Of Atom With Faqs
Because many identical RNA copies can be made from the same gene and each RNA molecule can direct the synthesis of many identical protein molecules cells can synthesize a large amount of protein rapidly when necessary.

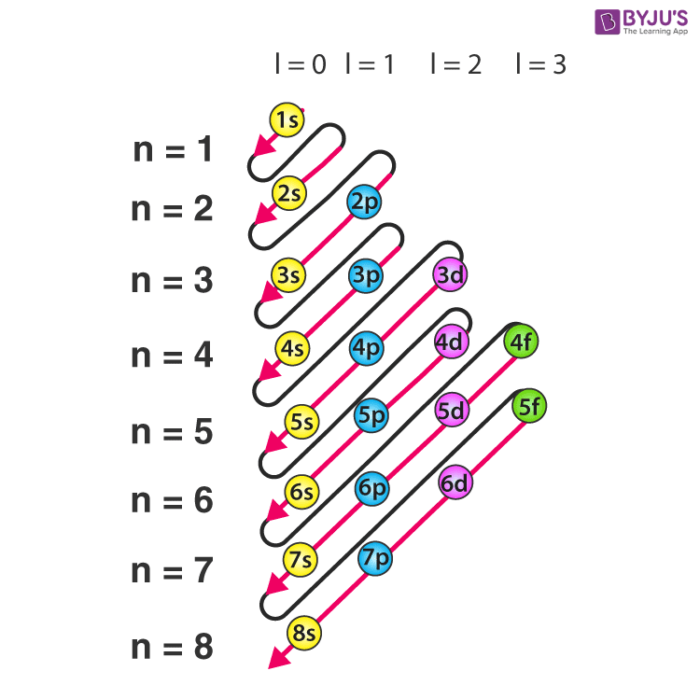
. An electron in an atom is defined by a set of four quantum numbers n the most important of which defines the main energy level known as a shell. The valence shell electron configuration of carbon is 2 s 2 2p x 1 2p y 1 2p z 0. Electron configuration was first conceived under the Bohr model of the atom and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.
The filling of electrons into different subshells also known as orbitals s p d f in an atom. Outside the battery electrons flow from one electrode to the other. Atoms can achieve this stable configuration by forming chemical bonds with other atoms.
There are two types of nodes they are angular and radial nodes. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration. Find out by adding single double or triple bonds and lone pairs to the central atom.
When metals bond with themselves they bond in a different way than when they bond. The structures of different types of star-shaped copolymers are illustrated above. Types of Radius with Respect to Types of Bonds.
Branched copolymers featuring differently structured main chains and side chains are known as graft copolymers. An electron shell is the set of allowed states that share the same principal quantum number n the number before the letter in the orbital label that electrons. However the tetrahedral structures of.
If this were the configuration used in covalent bonding carbon would only be able to form two bonds. In an orbital the number of nodal planes is equal to the azimuthal quantum number. Inside the circuit is closed by the electrolytes ions.
The nodal plane is the plane that passes through the nucleus on which the probability of finding an electron is zero. For atoms with partially filled d or f subshells these electrons are typically omitted from Lewis electron dot diagrams. To get a precise measurement of the radius but still not an entirely.
Explore molecule shapes by building molecules in 3D. Oxidation and Reduction at the Electrodes. Metals make up most of the elements in the periodic table around 80 and they are special.
This phenomenon can be explained by the Heisenberg Uncertainty Principle. This chemical bond can be formed either by gaining or losing an electrons NaCl MgCl2 or in some cases due to the sharing of an electron. The electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or molecular orbitals.
Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. An illustration detailing the structure of a graft copolymer made up of. Node is a region where the probability of finding the electron will be zero.
Determining the atomic radii is rather difficult because there is an uncertainty in the position of the outermost electron we do not know exactly where the electron is. Pronounced in French is a common analytical technique used specifically in the study of surfaces and more generally in the area of materials scienceIt is a form of electron spectroscopy that relies on the Auger effect based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. Explain why some molecules do not dissolve in water.
For example the electron dot diagram for iron valence shell configuration 4s 2 3d 6 is as follows. 8 electrons in the outermost shell thus symbolize a stable configuration. Transcription and translation are the means by which cells read out or express the genetic instructions in their genes.
Auger electron spectroscopy AES. In this case the valence shell would have six electrons- two shy of an octet. They consist of a multifunctional centre to which three or more polymer chains are attached.
Here the electrode reactions convert chemical energy to electrical energy. How does molecule shape change with different numbers of bonds and electron pairs. Then compare the model to real molecules.
The atoms having octet configuration ie. Different compounds having the same molecular formula are called isomers. When one s orbital and three p orbital from the same shell of atom mix together to form a new equivalent orbital then this is called sp³ hybridization.
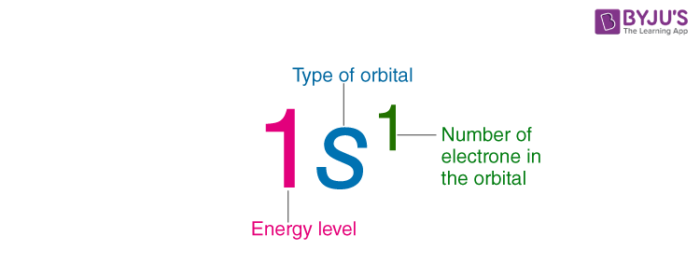
Electron Sea Model. Two materials with different electron affinities are used as electrodes. Electron Configuration is referred to as the distribution of electrons in an atoms orbitals.
But each gene can also be.

Electron Configuration Of Atoms Electrical4u
Electron Configuration Detailed Explanation Filling Of Orbital Representation Of Electronic Configuration Of Atom With Faqs
0 Comments